Description:
Explore fluid phase equilibria in mixtures through a comprehensive 7-hour video series. Delve into phase diagrams, Raoult's law, activity models, vapor-liquid-liquid equilibrium (VLLE), fugacity, and chemical potential. Learn to perform calculations for bubble and dew points, flash operations, and partial molar properties. Investigate non-ideal solutions, osmotic pressure, and the application of equations of state. Gain practical skills in using software tools like POLYMATH, Excel Solver, and COSMOtherm for thermodynamic calculations. Enhance understanding through interactive simulations and real-world examples, covering topics from basic concepts to advanced applications in chemical engineering thermodynamics.
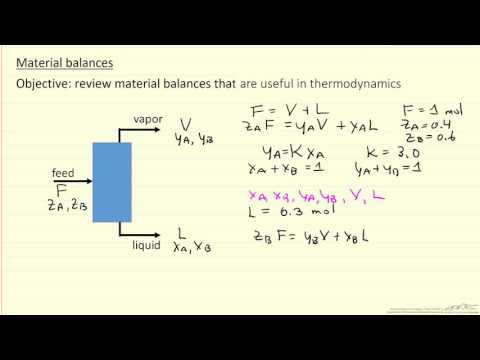
Thermodynamics: Fluid Phase Equilibria in Mixtures
Add to list
#Science
#Physics
#Thermodynamics
#Materials Science
#Phase Diagrams
#Phase Equilibrium
#Chemical Potential