Description:
Explore intermolecular forces in this comprehensive 46-minute chemistry video tutorial. Delve into hydrogen bonding, ion-ion interactions, dipole-dipole forces, ion-dipole interactions, London dispersion forces, and van der Waals forces. Learn through numerous examples and practice problems covering topics such as electrostatic forces, lattice energy, melting point comparisons, polar molecules, and charge separation. Understand the differences between intermolecular and intramolecular forces, as well as the relationship between polarizability and dispersion forces. Examine how to determine the strongest intermolecular forces in various compounds and explore the connection between boiling points and vapor pressure. Compare straight-chained and branched alkanes, analyze the polarity and water solubility of organic compounds, and investigate the effects of molar mass and electron count on overall intermolecular forces.
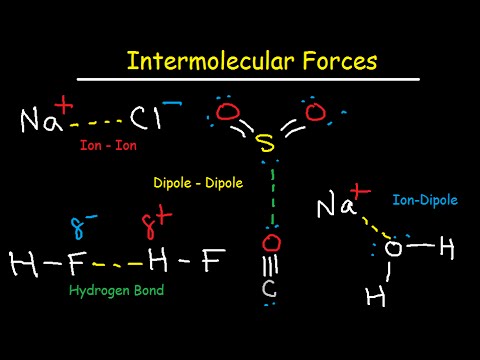
Intermolecular Forces - Hydrogen Bonding, Dipole-Dipole, Ion-Dipole, London Dispersion Interactions
Add to list