Description:
Learn how to draw coordinate bonds using the Valence Bond Approach and Lewis Dot Structure in this 11-minute chemistry tutorial. Explore step-by-step explanations of coordinate bonding in various compounds, including the hydronium ion (H3O+), ammonium ion (NH4+), the reaction between ammonia and boron trifluoride (NH3 + BF3), sulfur dioxide (SO2), and sulfuric acid (H2SO4). Gain a comprehensive understanding of coordinate bonds and their applications in chemical structures through clear explanations and visual representations.
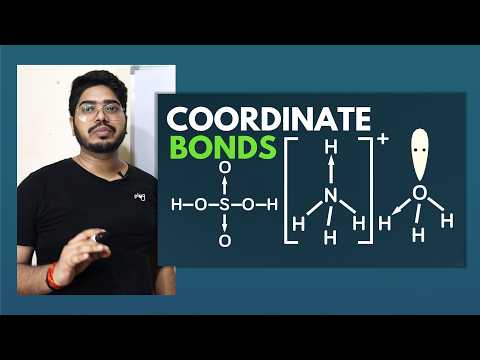
Coordinate Bonds - Drawing and Examples in Chemical Bonding
Add to list