Description:
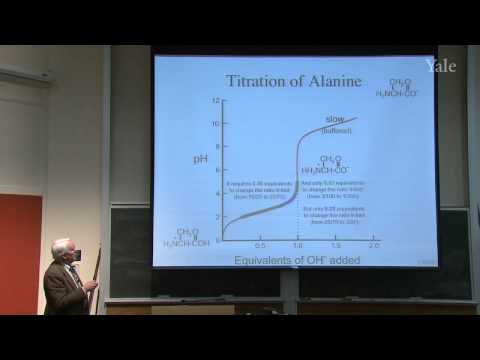
Explore the fundamental concepts of Brønsted acidity and nucleophilic substitution in this 47-minute lecture from Yale University's Freshman Organic Chemistry II course. Delve into the ionic dissociation of water and its relevance to Brønsted acidity. Examine how relative pKa values provide insights into energy-match, overlap, and resonance in ionic dissociation. Learn about the experimental determination of pKa values and buffering through the titration of alanine in water. Discover how the pKa scale can be extended using different solvents, offering a powerful tool for measuring various effects in organic chemistry. Gain a comprehensive understanding of nucleophilic substitution and its applications in 19th-century organic reactions and biochemical processes. The lecture covers solvent influence on ionic dissociation, Brønsted acidity as nucleophilic substitution at hydrogen, factors affecting pKa values, and the broader implications of nucleophilic substitution in organic chemistry.
Brønsted Acidity and the Generality of Nucleophilic Substitution
Add to list