Description:
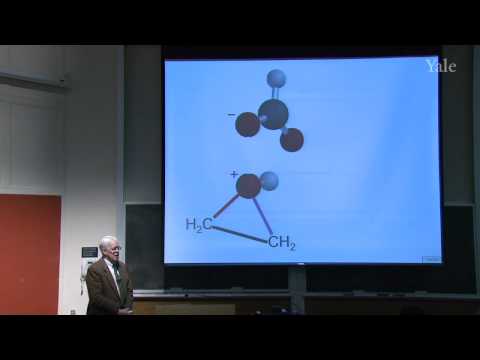
Explore the formation of three-membered rings in organic chemistry through this 51-minute lecture from Yale University's Freshman Organic Chemistry II course. Begin with a drill on the pinacol rearrangement mechanism before delving into molecular-orbital analysis of simultaneous electrophilic/nucleophilic attacks forming three-membered rings from alkenes. Practice using the curved-arrow formalism to describe electron-pair shifts. Examine two potential mechanisms for cyclopropane formation using the Simmons-Smith carbenoid reagent, with theoretical support for the one-step mechanism. Investigate epoxidation of alkenes by peroxycarboxylic acids, focusing on the concerted electrophilic/nucleophilic process. Discuss various paths to epoxides, their stereochemistry, scale, and commercial applications. Gain comprehensive insights into carbenoid reactions and epoxidation processes in organic synthesis.
Addition to Form Three-Membered Rings - Carbenoids and Epoxidation
Add to list