Description:
Explore the groundbreaking Bohr model of the atom in this 27-minute video lecture. Delve into Niels Bohr's pioneering work from 1913 that laid the foundation for quantum mechanics. Examine the limitations of Thomson's Plum Pudding model and Rutherford's Nuclear model before diving into Bohr's quantum model, which combines classical electrostatics with angular momentum quantization. Calculate the radii and energies of allowed electron orbits for the Hydrogen atom and study its visible emission spectrum. Gain insights into Bohr's influence on quantum physics development, covering topics such as alpha scattering, Bohr's postulates, Coulomb's Law, circular motion, energy levels, and the periodic table of emission spectra.
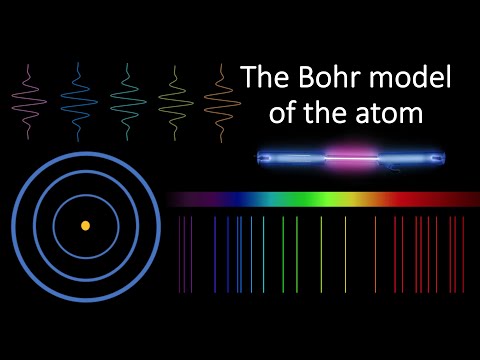
What Is the Bohr Model of the Atom?
Add to list
0:00 / 0:00